Discontinuation Notice
Instrument sales of the On-chip SPiS were discontinued in 2024. Please note that this instrument will be not available as soon as the stock runs out.
In recent years, single-cell analysis technology has been attracting attention. Single cell analysis requires accurate cell dispensing technology, but the lack of such technologies had been the bottleneck of research. Especially, cancer research involves vast stains of clones and the demand for analysis of each individual cancer cells as well as the determination of subtypes and genetic mutations are escalating rapidly.
Limiting dilution widely used for cell cloning is a simple and low-cost method, but the single cell dispensing accuracy is low and repeated re-cloning operation is required. In contrast, dedicated single cell analysis instruments are expensive and inconvenient for general purposes, while the accuracy is not at a satisfactory level.
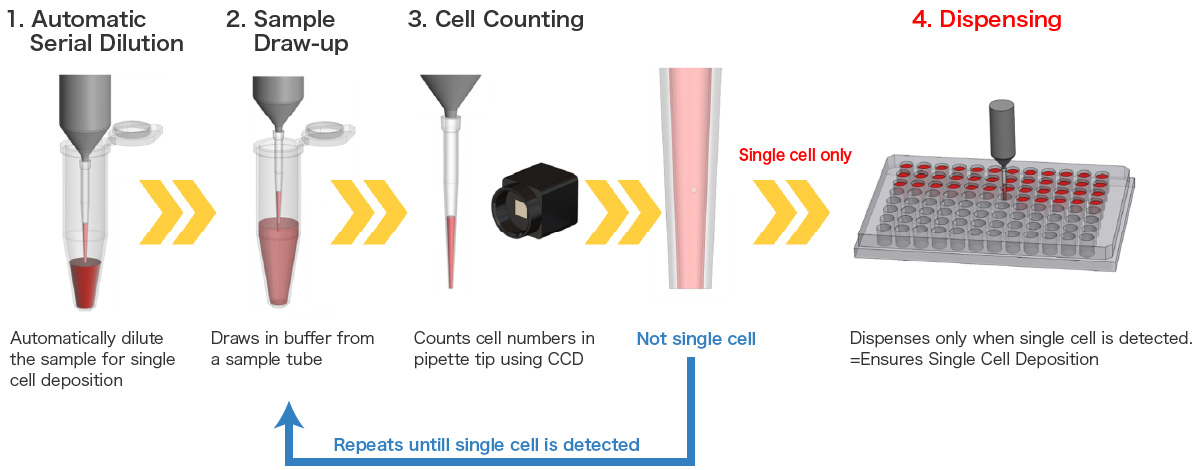
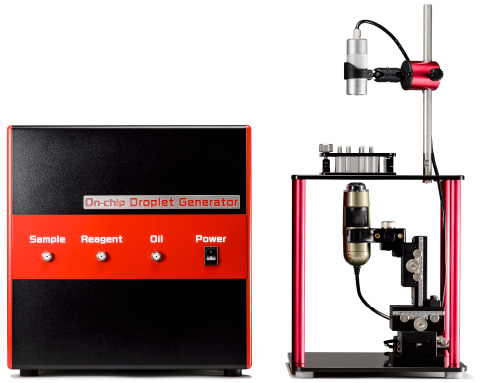
Therefore, On-chip Biotechnologies has developed On-chip SPiS in order to solve this problem. It is an easy-to-use instrument that allows dispensing of cells and large particles with single-cell/particle dispensing accuracy of over 90%.
- Easy and automatic operation
- Disposable pipette tip-based dispensing system
- Automatic dilution function + CCD camera image recognition
- Dispensing of sample sized up to 200 µm
Conventional methods
(example: the limiting dilution method: 0.3 cells/well on avarage)
On-chip SPiS
Workflow of On-chip SPiS
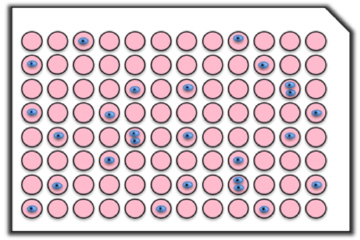
Accuracy and analysis speed of On-chip SPiS
Accuracy and analysis speed of single-cell dispensing
A549 cells were dispensed into 96 wells at 1 cell/well in 54 minutes. The number of wells with a single cell dispensed was 90, and cell proliferation was confirmed in 83 of them.
Dispensing time: 54 min/96 wells
| No. of cells dispensed | No. of wells (%) | No. of wells with proliferated cells (%) |
|---|---|---|
| 0 | 4 (4.2%) | ー |
| 1 | 90 (93.8%) | 83 (92.2%) |
| 2 | 2 (2.1%) | 2 (100%) |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| B | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| C | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 |
| D | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| E | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 |
| F | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 |
| G | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| H | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
*Each compartment represents a well, and the number shown in each compartment indicates the number of cells dispensed.
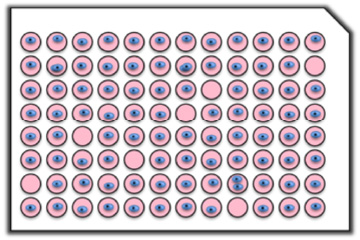
Confirmation of quantity-specified dispensing (ATP assay)
Quantity-specified dispensing
Cell type: PC9
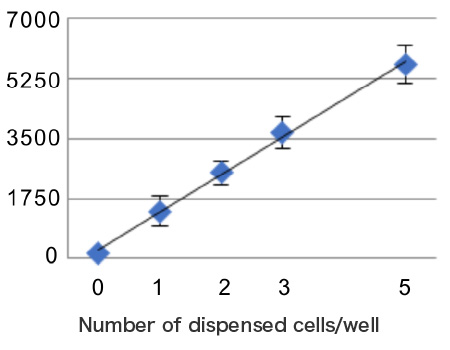
The number of cells dispensed per well was specified to 1,2,3, or 5 cells/well.
Verification of dispensing accuracy based on the ATP amount
PC9 cells were dispensed into 96-well plates at 1 cell/well. Single cell dispensing accuracy of about 90% was determined by measuring the amount of ATP in each well.
[ Dispensing accuracy of 90% ]
・A single cell was dispensed in each of 86 wells
・Two cells were dispensed in each of 10 wells
Applications
Single cell dispensing of knockout strains
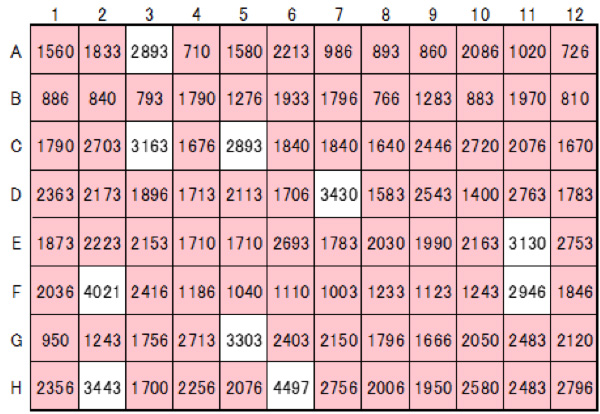
On-chip SPiS is useful for post-genome editing screening. A poliovirus (PV) receptor knockout cell line was generated using CRISPR-Cas9. A single cell was dispensed into each well of a well plate and cultured, followed by viral infection. As a result, 49 clones that acquired PV resistance were successfully established.
Collaboration with Dr. Koike, Tokyo Metropolitan Institute of Medical Science
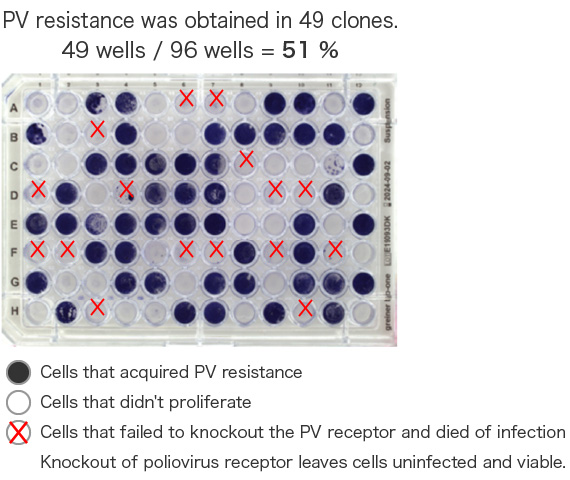
Collaboration with Dr. Koike, Tokyo Metropolitan Institute of Medical Science
HDR mutant clone selection
Transfected HEK293T cells were sorted by our microfluidic chip cell sorter (On-chip Sort) to obtain positive strains, and single cells were dispensed by On-chip SPiS. As a result, genome-edited clones were efficiently obtained one by one. In addition, clones with homozygous mutations were efficiently obtained.
*See also for details of On-chip Sort
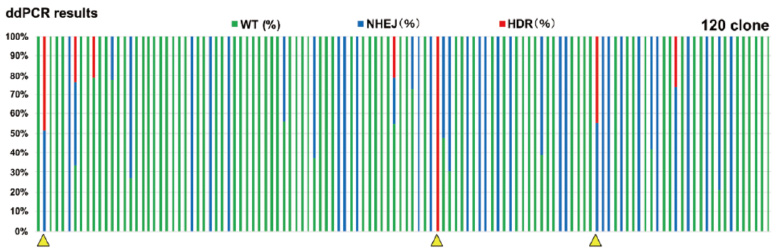
 Clones with the target heterozygous or homozygous mutations
Clones with the target heterozygous or homozygous mutations*If the allele frequency was less than 20%, it was removed as zero.
Dispenced cells:384
Obtained clones:120
Genome-editing occurred clones:41
Target HDR mutation clones:7
(Clones with homozygous HDR mutation) (1)
Collaboration with Dr. Miyaoka, Tokyo Metropolitan Institute of Medical Science
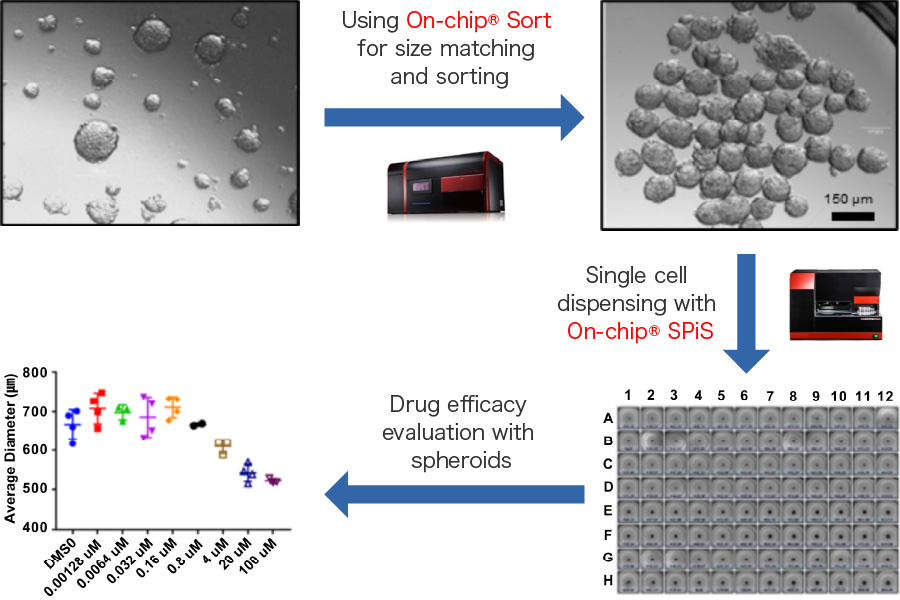
Dispensing of spheroids of different sizes for drug evaluation
The use of spheroids (cell masses) which can reproduce the biological environment has attracted attention in the evaluation of drugs such as anti-cancer agents. Uniform sized spheroids are necessary for highly accurate evaluation. Therefore, On-chip Sort was used to select spheroids of a specific size and On-chip SPiS was used to dispense the collected spheroids on to a well plate for fast and accurate drug efficacy evaluation.
Dispensing of gel microdrops (GMDs) containing bacteria
On-chip SPiS can dispense not only cells and large particles, but also GMDs (microdroplets solidified as gels). Bacteria grown from a single cell can form microcolonies inside the GMDs, which can be recognized by the CCD camera in On-chip SPiS. The use of GMDs is useful in screening and isolating environmental microbes and generation of mutant libraries.
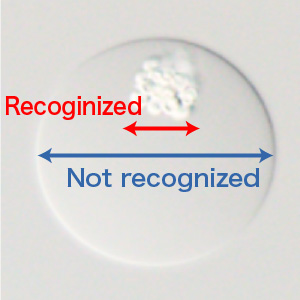
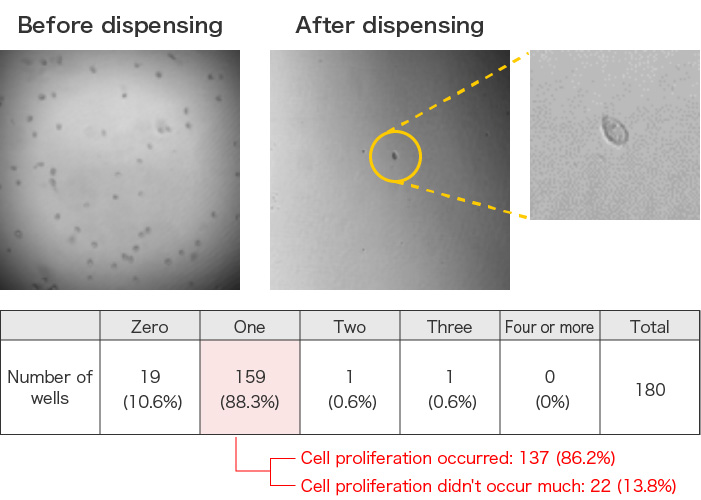
Dispensing and culture of swimming tetrahymena
Protozoan Tetrahymena were dispensed at 1 cell/well into a total of 180 wells and cultured for three days. Single cell dispensing was confirmed in 159 wells, 137 of which had cell proliferation. This result indicates that the system can dispense migrating cells with high accuracy.
Collaboration with Dr. Nakano, Tsukuba University
SPEC & Product Information
| Product name | On-chip SPiS |
|---|---|
| Product number | 70001 |
| Configuration | Main unit and control PC (Windows) |
| Main unit size | 610 x 365 x 450 / W x D x H (mm) |
| Dispensing method | Disposable pipette tips |
| Recognition method | Image recognition by a camera with built-in 5-megapixel resolution CMO sensor |
| Tip volume | 0.2μL |
| Sample | Cells, pollen, protists, cell aggregates, beads, gel micro drops, etc. |
| Recognition size | 10 – 200 μm 10–200 μm (cultured cells are recognized) |
| Dispensing accuracy | ≥ 90% (depending on sample conditions) |
| Biosafety | Installable in a biosafety cabinet |
| Damage to samples | Low damage to samples such as cells |
| Stirring method | Automatic stirring with the disposable pipette tip |
| Solution | Water, culture media, seawater, etc. |
| Processing speed | 60 min/96 wells (changeable with setting conditions and sample conditions) |
| Supported plate | 96-well plates, 384-well plates |
| Power input | AC 100-120V, 50/60Hz |
| Power consumption | 1.0A typ (ACIN 100V) |


